How To Find How Many Neutrons In An Element: A Simple Guide For Science Enthusiasts
Ever wondered how to figure out the number of neutrons in an element? Well, you're in the right place, my friend. Whether you're a student trying to ace your chemistry test or just someone curious about the world of atoms, this guide has got you covered. We'll break down the process step by step so it's as easy as pie. So grab a cup of coffee, sit back, and let’s dive into the fascinating world of neutrons!
Science can sometimes feel like a foreign language, but trust me, it doesn’t have to be that way. Understanding neutrons isn’t rocket science—well, actually, it kind of is, but you get the point. In this article, we’ll explore the basics of atomic structure and explain how to calculate the number of neutrons in any element. By the end of this, you’ll be the neutron whisperer among your friends!
Before we jump into the nitty-gritty, let’s quickly remind ourselves why neutrons are such a big deal. They’re like the unsung heroes of the atom, holding everything together without stealing the spotlight from protons and electrons. So, are you ready to uncover the secrets of neutrons? Let’s get started!
Read also:How To Deposit Money In Majority A Comprehensive Guide
Table of Contents
- Understanding the Basics of Atomic Structure
- What is the Mass Number and How Does It Help?
- The Role of Atomic Number in Calculating Neutrons
- The Formula to Find Neutrons
- Examples of Calculating Neutrons in Common Elements
- How Isotopes Affect the Number of Neutrons
- Using Online Tools to Simplify the Process
- Real-World Applications of Neutron Knowledge
- Tips and Tricks for Memorizing Neutron Calculations
- Wrapping Up: Mastering the Art of Neutron Calculation
Understanding the Basics of Atomic Structure
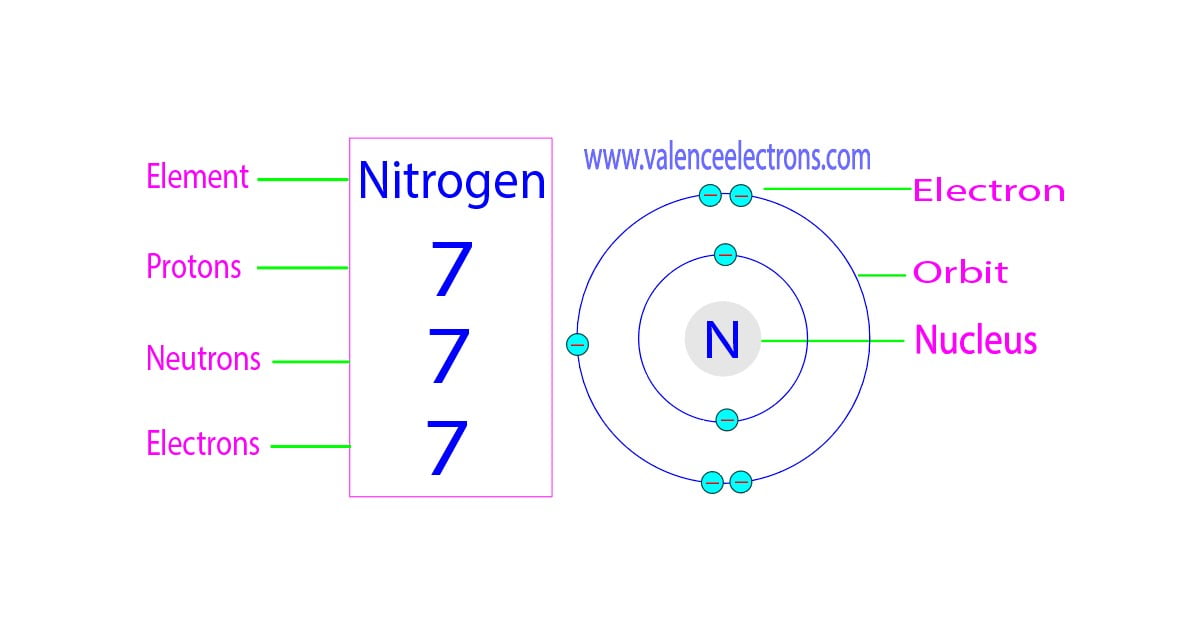
Alright, let’s start with the fundamentals. Every element is made up of tiny building blocks called atoms, and these atoms consist of three main particles: protons, neutrons, and electrons. Think of them as the atomic dream team. Protons carry a positive charge, electrons are negatively charged, and neutrons? Well, they’re neutral, which is why they’re called “neutrons.”
Now, here’s the thing: protons and neutrons hang out in the nucleus, which is like the control center of the atom. Electrons, on the other hand, orbit around the nucleus like little satellites. To figure out how many neutrons are in an element, we need to focus on the nucleus and its contents. Stick with me, because it gets interesting!
Breaking Down the Nucleus
So, what’s inside the nucleus? Protons and neutrons, right? The number of protons determines the identity of the element. For example, hydrogen has one proton, helium has two, and so on. But neutrons? They’re a bit more flexible. The number of neutrons can vary, which is where isotopes come into play—but we’ll get to that later.
What is the Mass Number and How Does It Help?
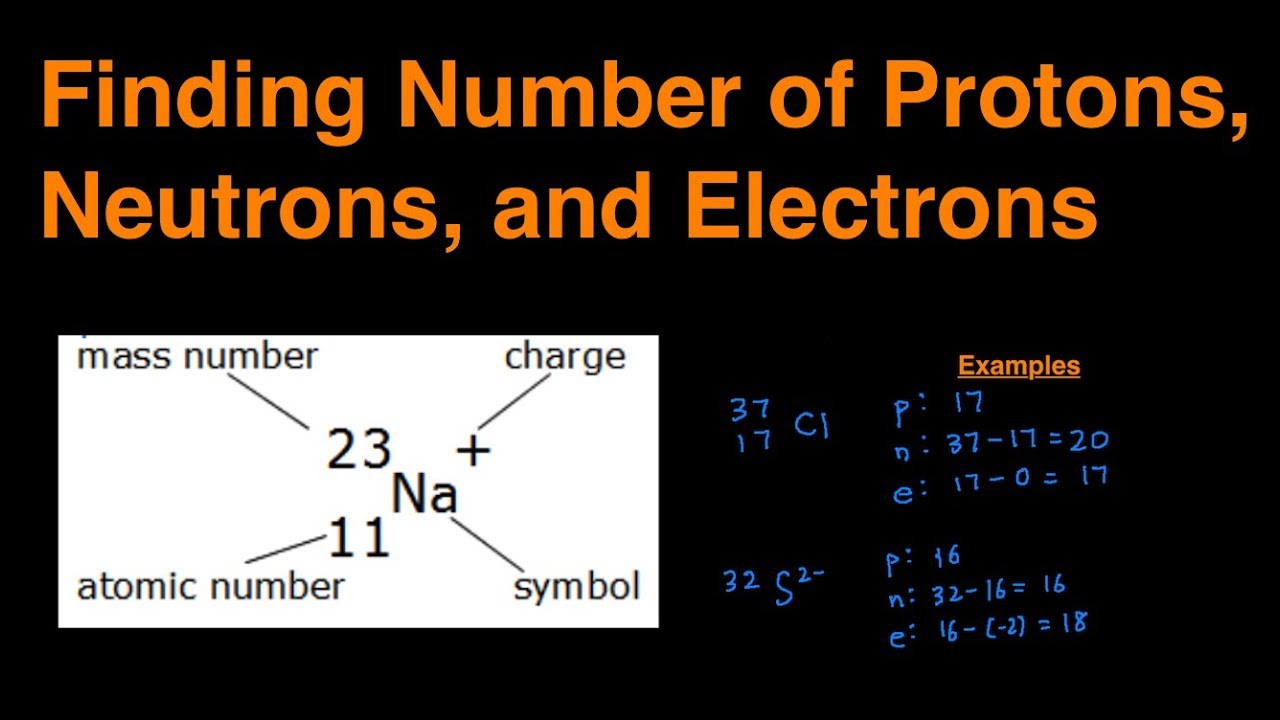
Here’s where things start to click. The mass number of an element is basically the sum of the protons and neutrons in its nucleus. It’s like a census for the nucleus, counting all the residents. Why is this important? Because once you know the mass number and the atomic number (the number of protons), you can calculate the number of neutrons.
Let’s break it down: Mass Number = Number of Protons + Number of Neutrons. See? Not so complicated after all. The mass number is usually written as a superscript next to the element’s symbol. For example, carbon-12 means the mass number is 12.
Why Does the Mass Number Matter?
The mass number gives us a clear picture of the total number of particles in the nucleus. It’s like knowing the size of your team before heading into a game. Without the mass number, we’d be flying blind when it comes to calculating neutrons. So, always keep an eye out for that little number!
Read also:99 Cent Apple Charge Whats The Deal And Why You Need To Know
The Role of Atomic Number in Calculating Neutrons
Now let’s talk about the atomic number. This is the number of protons in an atom, and it’s what defines the element. For instance, if an atom has six protons, it’s carbon. If it has eight protons, it’s oxygen. Simple, right?
Here’s the kicker: the atomic number is constant for a given element. That means no matter how many neutrons an atom has, the number of protons stays the same. This consistency makes it super easy to identify elements and calculate neutrons.
Connecting the Dots
So, if we know the mass number and the atomic number, we can calculate the number of neutrons. It’s like solving a little puzzle. Just subtract the atomic number from the mass number, and voila! You’ve got your neutron count.
The Formula to Find Neutrons
Let’s put it all together. The formula to find the number of neutrons in an element is:
Number of Neutrons = Mass Number - Atomic Number
It’s as simple as that. No fancy equations, no complex math. Just a straightforward subtraction problem. Now, let’s see this formula in action with some examples.
Examples of Calculating Neutrons in Common Elements
Time for some real-world examples. Let’s calculate the number of neutrons in a few common elements:
- Carbon-12: Mass Number = 12, Atomic Number = 6 → Neutrons = 12 - 6 = 6
- Oxygen-16: Mass Number = 16, Atomic Number = 8 → Neutrons = 16 - 8 = 8
- Iron-56: Mass Number = 56, Atomic Number = 26 → Neutrons = 56 - 26 = 30
See how easy that was? With just a little bit of information, you can figure out the neutron count for any element.
What About Rare Elements?
Even for rare or exotic elements, the formula stays the same. Just plug in the mass number and atomic number, and you’re good to go. Whether it’s uranium or plutonium, the process is consistent and reliable.
How Isotopes Affect the Number of Neutrons
Now, let’s talk about isotopes. Isotopes are versions of the same element with different numbers of neutrons. For example, carbon-12 and carbon-14 are both carbon, but they have different neutron counts. Carbon-12 has six neutrons, while carbon-14 has eight.
Why does this matter? Because isotopes can have different properties and uses. Some isotopes are stable, while others are radioactive. Understanding isotopes helps scientists in fields like medicine, energy, and even archaeology.
Spotting Isotopes in Action
Isotopes are everywhere! Radiocarbon dating, for instance, relies on the decay of carbon-14 to estimate the age of ancient artifacts. Medical imaging often uses isotopes to create detailed pictures of the body. So, next time you hear about isotopes, remember—they’re not just theoretical; they’re practical!
Using Online Tools to Simplify the Process
If you’re not a fan of manual calculations, don’t worry. There are tons of online tools and apps that can help you find the number of neutrons in an element. Just enter the element’s name or symbol, and the tool will do the math for you.
But here’s a pro tip: even if you use a tool, it’s still good to understand the basics. That way, you can double-check the results and impress your friends with your scientific knowledge.
Which Tools Are the Best?
Some of the most reliable tools include the Periodic Table of Elements by WebElements, ChemSpider, and the NIST Chemistry WebBook. These resources are trusted by scientists and students alike, so you know you’re getting accurate information.
Real-World Applications of Neutron Knowledge
Knowing how to calculate neutrons isn’t just academic; it has real-world applications. For example, nuclear power plants rely on the behavior of neutrons to generate energy. Chemists use neutron calculations to study molecular structures, and physicists use them to explore the mysteries of the universe.
Even everyday technologies, like smoke detectors and MRI machines, depend on our understanding of neutrons. So, the next time you flip a light switch or get an MRI, remember that neutrons played a role in making it possible.
Neutrons in Everyday Life
From powering our homes to diagnosing medical conditions, neutrons are an essential part of modern life. By understanding how to calculate them, you’re not just learning science; you’re unlocking the secrets of the world around you.
Tips and Tricks for Memorizing Neutron Calculations
Let’s face it: memorizing formulas can be tricky. But don’t worry, I’ve got some tips to help you remember how to calculate neutrons:
- Think of the mass number as the total “weight” of the atom.
- Remember that the atomic number is the number of protons, which never changes for a given element.
- Practice with different elements to reinforce the formula.
- Use flashcards or apps to quiz yourself regularly.
With a little practice, calculating neutrons will become second nature. Trust me, it’s worth the effort!
Making It Stick
Repetition is key when it comes to memorization. Try explaining the process to a friend or teaching it to someone else. Teaching reinforces your own understanding and helps the information stick in your brain.
Wrapping Up: Mastering the Art of Neutron Calculation
And there you have it, folks! A comprehensive guide to finding the number of neutrons in an element. From understanding atomic structure to calculating isotopes, we’ve covered it all. Remember, science doesn’t have to be intimidating. With the right approach, anyone can master the art of neutron calculation.
So, what’s next? Why not try calculating neutrons for a few elements on your own? Or maybe share this article with a friend who’s curious about science. Whatever you do, keep exploring and keep learning. After all, the world of science is full of wonder, and neutrons are just the beginning!
Thanks for reading, and don’t forget to leave a comment or share your thoughts. Until next time, stay curious!
Minister Of Winter: A Deep Dive Into The Realm Of Cold Weather Politics
How To Start A Pocket Bike: A Beginner's Guide For Thrill-Seekers
Cuidado Bebe Suelto 2: A Wild Ride For Parents And Kids
Protons, Neutrons, Electrons For Phosphorus (P, P3), 58 OFF

How Many Neutrons Does This Element Have
Which Element Has 15 Neutrons